Background
Sickle Cell Disease (SCD) is a genetic disorder caused by an HBB gene mutation and resulting structural variant haemoglobin (HbS) that polymerises upon deoxygenation, leading to a sickled morphology of red blood cells ( Chakravorty et al., Arch Dis Child 2015). This causes acute pain episodes, known as vaso-occlusive crises (VOC), that can result in severe end-organ damage in the long-term and a shortened lifespan ( Jang et al., J Transl Med 2021). Responsible for an estimated 95% of SCD hospitalisations and as a key predictor of death ( Darbari et al., Eur J Haematol 2020, Ballas et al., Am J Hematol 2005), it is critical to find new ways to drive early detection of VOCs in order to support preventative interventions.
Aims
This work aimed to develop a machine learning (ML) algorithm capable of predicting the potential onset of a VOC, using physiological data captured longitudinally by a wearable smartwatch, as well as patient-reported outcomes (PROs) entered via a specialised mobile phone application.
Methods
Following informed patient consent, participants gained access to a mobile phone application, or “digital patient wallet”, encompassing their health data and access to a PRO entry portal ( Summers et al., HemaSphere 2023). This allowed for daily recording of PROs including EQ-5D-5L, pain, mood, and fatigue scores, alongside self-reported VOCs. Participants were also provided with a Withings ScanWatch to be worn day and night, capturing physical activity, sleep quality, and heart rate data in an automated manner. Medical record data including healthcare utilisation, pathology, and demographic data was obtained through participant completion of a Subject Access Request form.
Data for a snapshot cohort of 186 patients over a 1-month period (May-June 2023) were analysed through an ensemble of ML models: gradient boosting machine, neural network, and k-means clustering. All collated metrics were fed into the model, with the exclusion of hospitalisation as an 'a posteriori' variable, to ensure predictions were based on appropriate variables and not strengthened by self-confirming factors. The resulting predicted likelihood of a VOC for each participant was calculated on each day and given as a scale from 0-100%, with a likelihood of 75% or higher deemed to be a predicted VOC. This was then assessed against the true incidence of the participants' self-reported VOCs.
Results
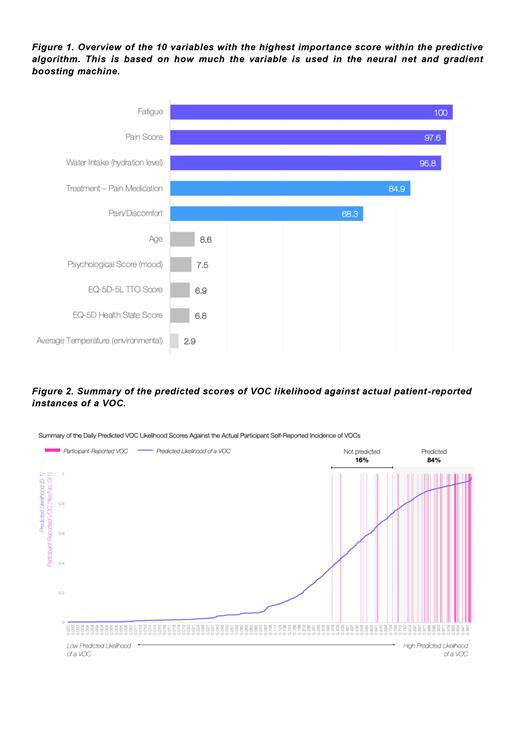
The mean age was 36 (SD 11.6) years, and most patients were female (70%). The HbSS genotype was most common (75%), followed by HbSC (16%), and HbS Beta + Thalassemia (5%). 82,804 datapoints were collated for all participants across this period, encompassing 90 different variables. 69 instances of a VOC were self-reported by patients during the period of analysis, 58 (84%) of which were accurately predicted by the algorithm. 2,366 instances of no VOC were self-recorded, giving a negative predicted value of 83% (1,958/2,366 predicted). Further breakdown by the predicted likelihood score is displayed in Figure 2, highlighting the spread of individually calculated VOC likelihood scores per day against the incidence of patient-reported VOCs.
Summary/Conclusions
This work provides an important translation of daily trackable metrics through a remote, digital ecosystem, to the real-world prediction of potential VOC events in SCD. The purpose of this algorithm is, at its core, to act as a pre-emptive warning system from which proactive behavioural changes or interventions can be enacted. As such, the prediction of additional potential risk scores above the 75% threshold reflects an approach of ‘over-predicting’ considered to be a preferable strategy for the algorithm, given the severe nature of VOCs and the health impacts of missing a potential event.
Our ongoing aim is to further develop the algorithm and its contributing metrics against medical data, in addition to patient self-reported events, to further optimise the predictor. This includes the curation of core metrics requiring minimal patient input, in order to reduce missing datapoints across those that feed into the algorithm and thereby reduce unpredicted VOCs. Ultimately, we hope to provide an early alert system that will help patients to identify their VOCs sooner, and thus action the next steps that may prevent possible hospital admissions.
Disclosures
Summers:Pfizer Ltd: Other: Employed by Sanius Health, who have a financial work contract with the company. Agrippa:Pfizer Ltd: Consultancy. Lugthart:GBT: Other: Conference attendance. Anie:Pfizer Ltd: Honoraria. Telfer:Terumo: Speakers Bureau; Kyowa Kirin Limited: Other: Investigator-led funding; Napp Pharmaceuticals: Other: Clinical trial activity; Celgene: Other: Clinical trial activity; Apopharma: Other: Clinical trial activity, Speakers Bureau; Pfizer: Other: Advisory board; Clinical trial activity; Data monitoring committee; ; Novartis: Other: Advisory board; Clinical trial activity; ; bluebird bio: Other: Advisory board; Investigator-led funding;.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal